|
Components of Cells and Batteries
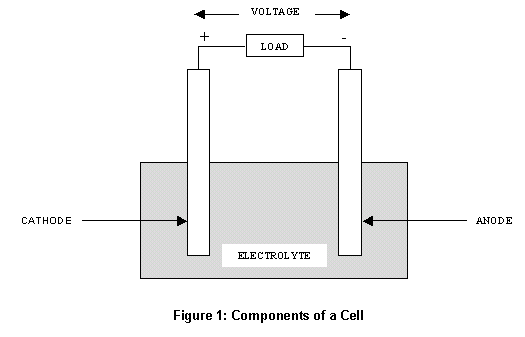
Cells are comprised of 3 essential components.
-
The Anode is the
negative or reducing electrode that releases electrons to the
external circuit and oxidizes during and electrochemical reaction.
-
The Cathode is
the positive or oxidizing electrode that acquires electrons from the
external circuit and is reduced during the electrochemical reaction.
-
The
Electrolyte is the medium that provides the
ion transport mechanism between the
cathode and anode of a cell. Electrolytes are often thought of as
liquids, such as water or other solvents, with dissolved salts,
acids, or
alkalis that are required for
ionic conduction. It should however
be noted that many batteries including the conventional (AA/AAA/D)
batteries contain solid electrolytes that act as ionic conductors at
room temperature.
Considerations in selection of Cathode,
Anode and Electrolyte
Desirable properties for
anode, cathode, and electrolyte materials are noted below.
Anode material should exhibit the following properties
Cathode material should exhibit the following properties
-
Efficient oxidizing agent.
-
Stable when in contact with electrolyte
-
Useful
working
voltage
-
Ease of fabrication
-
Low cost
-
Metallic oxides such as are often used as cathode
materials
The most desirable
anode-cathode material combinations are those that result in light-weight
cells with high voltage and capacity. Such combinations may not always be
practical as a result of extenuating factors such as material handling
difficulty, reactivity with other cell components, difficulty of
fabrication,
polarization
tendencies, and cost prohibitive materials.
Electrolytes should exhibit the following
properties
-
Strong ionic conductivity
-
No electric conductivity
-
Non-reactivity with electrode materials
-
Properties resistance to temperature fluctuations
-
Safeness in handling
-
Low cost
-
Aqueous
solutions such as dissolved salts, acids, and alkalis are often
used as electrolytes
Home |
Previous | Next |
